High speed confocal 4D imaging, spectral analysis, super-resolution imaging, imaging multiprotein complexes by multiplexing FRET/FLIM
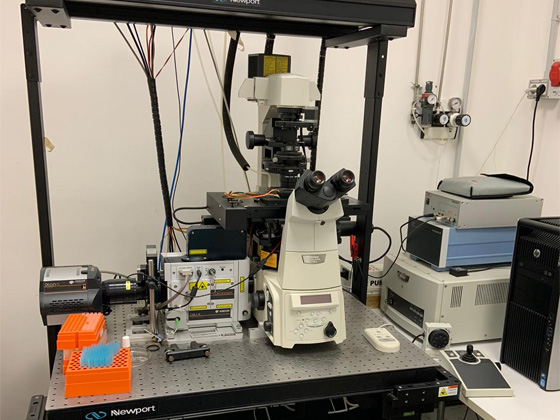
The facility is equipped with two laser scanning confocal microscopes, one spinning disc confocal microscope and a structured-illumination based microscope. All the microscopes are equipped with temperature and CO2 control modules necessary for live imaging studies. The IEOS users will be provided batch training once in a year in the use of confocal microscopes and the apotome microscope and if sufficient numbers of people are present more additional courses will be considered. For the use of other microscopes individual training will be provided in case of need.
Services provided
- Live-cell imaging (time-lapse microscopy), for monitoring intracellular organelles and protein dynamics in time and in 3D space;
- Video Microscopy including Fluorescence recovery after photobleaching (FRAP) and Fluorescence Loss in Photobleaching (FLIP) for quantitative evaluation of protein mobility within organelles, rates of inter-organelle exchange, and protein binding kinetics;
- Fluorescence resonance energy transfer (FRET) and Fluorescence Lifetime Imaging (FLIM) , to evaluate the strength and intracellular topology of protein-protein interactions, and monitor intracellular dynamics of small signalling molecules and protein activities;
- Lambda imaging;
- Super resolution imaging (G-STED);
- Cell microinjection, for acute modulation (activation or inhibition) of protein activities;
- Image analysis station equipped with Zen off line analysis program, AxioVision off line analysis program, Leica image analysis suite, Image J, Cell profiler and Metamorph image analysis software.