Cell Physiology
Keywords: Golgi apparatus; self-organization; Glycosylation; evolution; Imaging
Group leader: S. Parashuraman
Contacts:
Phone: +39 0816132283
Lab: +39 08161325206
Email: raman.sp@cnr.it
Organization, spatial and temporal, is a fundamental feature of life. The form and function of an organism is determined by the organization of its molecular constituents. The last decade had exploded with “omics” technologies that have given us the parts list of many organisms including our own. The next challenge is in elucidating the organizing principles that dictate the interactions between these constituents leading to the emergence of life as we see it.
We use the secretory pathway of the eukaryotic cell as a model system to understand these principles. The eukaryotic cell consists of intracellular membrane-bound compartments of which the secretory pathway forms a major part. Nearly 1000 proteins are localized to it and about one-third of the proteins encoded by our genome depend on it for their biosynthesis and proper localization. Apart from the proteins, the secretory pathway also contributes to the biosynthesis of most of the lipids present in eukaryotic cell and polysaccharides (like scales in the case of algae).
To get a systems perspective of the secretory pathway we pursue answers to these following questions:
1) How do the constituents of the pathway interact to promote the specific morphological and functional features to the secretory pathway?
2) What are the contributions of the pathway to the cellular physiology ?
3)How are the secretory pathway functions regulated ?
4) What are the physiological and evolutionary significance of such an organization?
We study the mammalian Golgi apparatus to answer these questions and we do so by actively following these research directions:
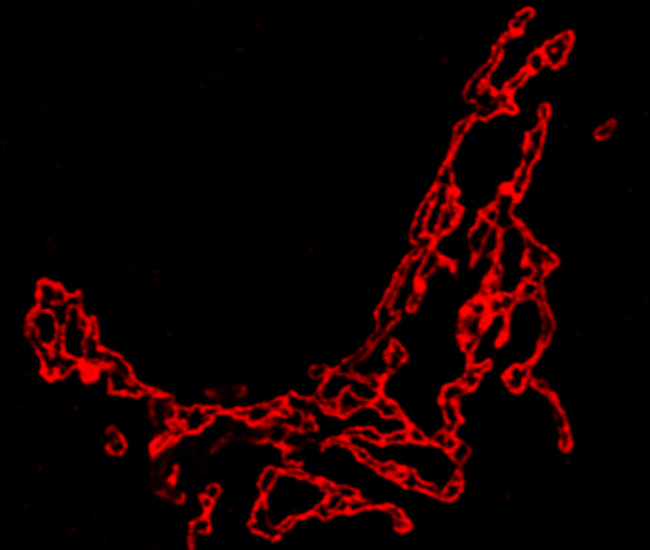
1) Understanding the functional organization of the Golgi: The Golgi apparatus is a dynamically stable compartment with well-differentiated molecular and/or structural zones. We would like to understand how this organization contributes to the transport and processing (glycosylation) functions of the organelle. To this end, we are building a molecular map of the Golgi apparatus and are interrogating the contribution of the specific spatio-temporal molecular organization to the functioning of the organelle.
2) Regulation of the secretory pathway: Here we focus on the regulation of the initial stages of the secretory pathway viz. the protein biosynthesis, folding and degradation in the Endoplasmic reticulum. Using the disease model of Cystic Fibrosis, we had recently found a set of regulatory pathways that regulate the ER associated degradation of proteins. We further exploring to understand how these pathways act and if they can be of therapeutic utility.
3) Functional interaction of Secretory pathway with other modules of the cell: The secretory pathway does not function in isolation and is integrated with other cellular functions (modules) of the cell. We are exploring and defining such relationships, using transcriptomics, in order to create an integrated functional map of the cell (from the perspective of the secretory pathway).
Golgi Apparatus Regulates Plasma Membrane Composition and Function.
Agliarulo I, Parashuraman S. Cells. 2022 Jan 22;11(3):368.
GRASP55 regulates intra- Golgi localization of glycosylation enzymes to control glycosphingolipid biosynthesis. Pothukuchi P, Agliarulo I, Pirozzi M, Rizzo R, Russo D, Turacchio G, Nüchel J, Yang JS, Gehin C, Capolupo L, Hernandez-Corbacho MJ, Biswas A, Vanacore G, Dathan N, Nitta T, Henklein P, Thattai M, Inokuchi JI, Hsu VW, Plomann M, Obeid LM, Hannun YA, Luini A, D'Angelo G, Parashuraman S. EMBO J. 2021 Oct 18;40(20):e107766.
Translation of genome to glycome: role of the Golgi apparatus. Pothukuchi P, Agliarulo I, Russo D, Rizzo R, Russo F, Parashuraman S. FEBS Lett. 2019 Sep;593(17):2390-2411.
Visualizing sphingolipid biosynthesis in cells. Parashuraman S, D'Angelo G. Chem Phys Lipids. 2019 Jan;218:103-111.
Unravelling druggable signalling networks that control F508del-CFTR proteostasis. Hegde RN, Parashuraman S, Iorio F, Ciciriello F, Capuani F, Carissimo A, Carrella D, Belcastro V, Subramanian A, Bounti L, Persico M, Carlile G, Galietta L, Thomas DY, Di Bernardo D, Luini A. Elife. 2015 Dec 23;4:e10365.

Researcher:
Marinella Pirozzi m.pirozzi@ieos.cnr.it
Ilenia Agliarulo i.agliarulo@ieos.cnr.it
PhD Student:
Antonietta Esposito a.esposito@ieos.cnr.it


European Commission launched the Euro-Bioimaging ERIC - A gateway to European imaging excellence.
The European Commission has officially established Euro-BioImaging – which provides life scientists with open access to a broad range of technologies and resources in biological and biomedical imaging – as a European Research Infrastructure Consortium (ERIC1). Imaging technologies have a central role in driving fundamental research and applied innovations in both biological and biomedical research. These technologies help a very broad user and research community to reach breakthrough biological discoveries and to proceed with translation into innovations in the fields of medicine, diagnostics, drug development, biotechnology, and molecular ecology.
https://www.cnr.it/en/news/9031/european-commission-launched-the-euro-bioimaging-eric-a-gateway-to-european-imaging-excellence

An insight into the Italian Advanced Light Microscopy Node
https://www.cnr.it/en/news/8870/an-insight-into-the-italian-advanced-light-microscopy-node


